Which of the Following Represents a Beta Particle
Select the best answer from the choices provided t f. 1an alpha particle 2a beta particle 3a neutron 4a positron 2Which nuclear emission has the greatest mass and the least penetrating power.

Alpha And Beta Adrenergic Receptors Catecholamine Receptors Related Keywords Suggestions Catecholamine
A β particle is emitted a.

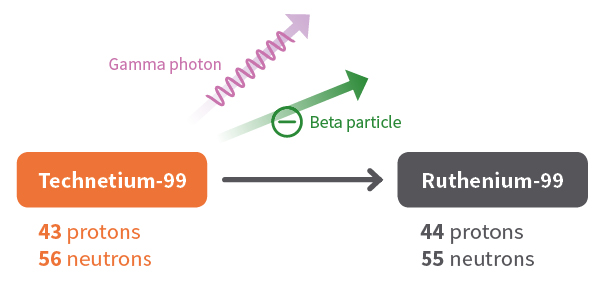
. The beta particle is a high-speed electron when it is a β- decay and a positron when it is a β decay. 242 4 1 245. Beta particles are used to treat health conditions such as eye and bone cancer and are also used as tracers.
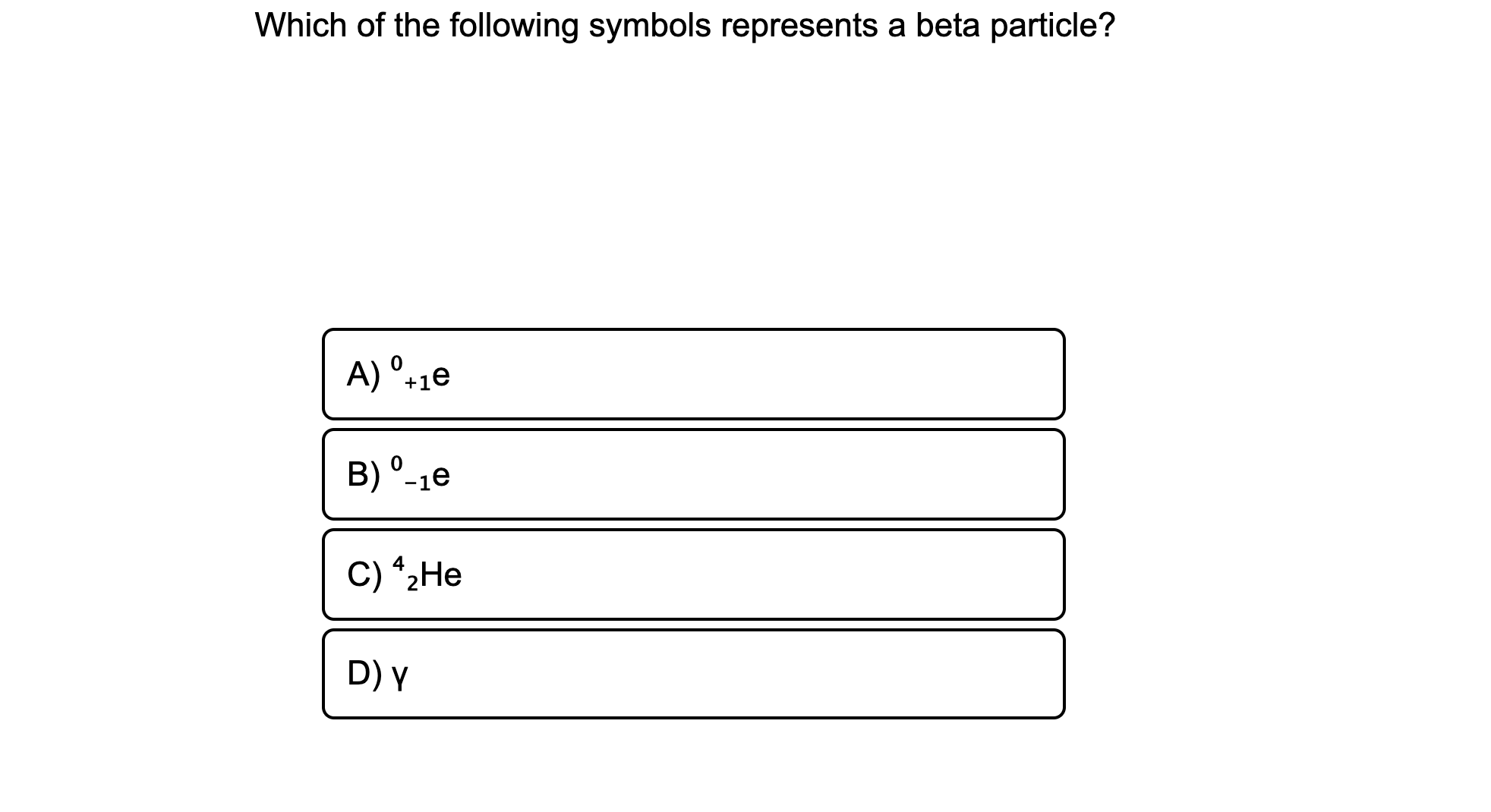
A 1e B -1e C 2He D Y. What are the uses of beta decay. Lymphocytes known as blastocysts make antibodies that fight infection.
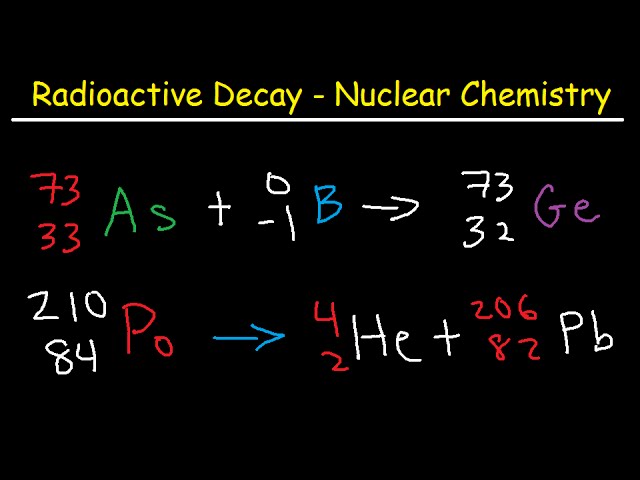
A chain reactions is started when 235 v92 U is bombarded with a neutron it will then undergo nuclear decay in which. O 98 6 104 15 8 1 o 87 85 239 93 Np-239N OB. Correct option is A 1.
1224Mg -10e Where e represents the beta particle which can also be viewed as an electron. The atomic number increases by one and the mass number remains unchanged c. The atomic number decreases by two and the mass number decrease by four b.
Heres a video to give more explanation. A gamma ray b. Reaction in which X represents a nuclide.
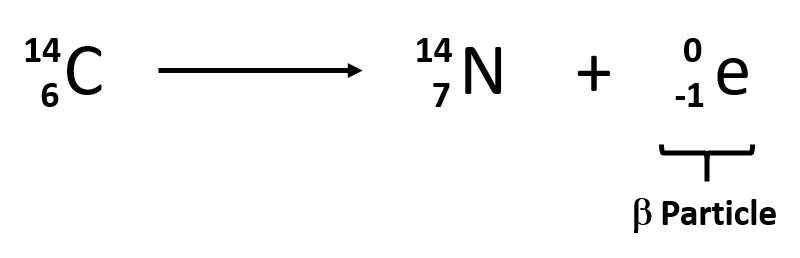
1 high speed neutrons are released to bombard other 235. 1 2 1H 2 1H -- 4 2He 2 14 6C -- 0-1e 14 7N 3 238. Beta particles are emitted in the process of beta decay.
Answer 1 of 4. B is untrue about a beta plus aka positron. Which of the symbols represent a beta particle.
A beta particle can be in form of an electron or a positron. An alpha particle c. When the equation is balanced correctly which particle is represented by X.
Which of the following represents a beta particle. The atomic number increases by one and the mass number remains unchanged c. What changes occur to the atomic number and mass of a nucleus during each of the following decay scenarios.
What changes occur to the atomic number and mass of a nucleus during each of the following decay scenarios. In the nuclear equation 23290 Th ---- 88228 Ra X the letter X represents. C is untrue in the sense that while a beta minu.
A ⁰₁e B ⁰₁e C ⁴₂He D γ. The atomic number and mass number remain unchanged. A beta particle d.
Up to 24 cash back Which of the following decay products will be repelled by a positively charged surface. Chemistry questions and answers. This problem has been solved.
In the reaction 23892U ---- X 24 He the. The carbon-14 atoms undergo beta-minus decay electron emission and produce a beta particle and a nitrogen-14 atom. Unfortunately the question is not specific enough.
Here a neutron of carbon is converted into a proton and the emitted beta particle is an electron. Which of the following represents an example of beta particle emission. Since it has a negative charge -1 a negative sign is usually included on the top right of the symbol as shown below.
Which equation represents a fusion reaction. The equation for the negative beta decay of 24Na is. 1alpha particle beta particle gamma ray 2alpha particle gamma ray beta particle 3gamma ray beta particle alpha particle.
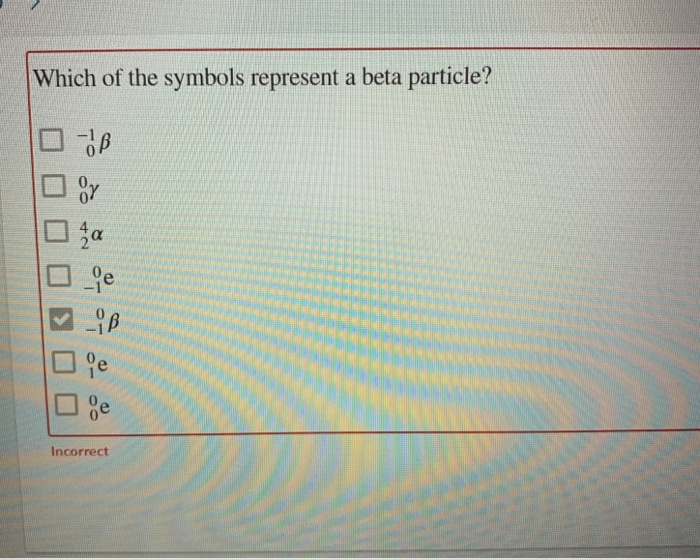
22Which one of the following is a correct representation of a beta particle. Option e is not about a beta particle it is about options so it is irrelevant. OB Oy za _e LiB lie ge Incorrect.
A is certainly untrue about ANY beta particle. Solution for Which of the following symbols represents a beta particle. He 4 is a fusion reaction because here two smaller nuclei fuse together to form a single stable nuclei.
What symbol is used for a beta particle. A 0 1 e B 0-1 e C 1 0 β D 4 2 e E 2 4 β 22 23Which one of the following processes results in an increase in the atomic number. An alpha particle c.
When cobalt-60 undergoes nuclear decay it emits 1 a positron 2 a neutron 3 a beta particle 4 an alpha particle 23. Which of the following will have the greatest amount of charge relative to its mass that is the greatest charge-to-mass ratio. The atomic number and mass number remain unchanged.
A beta particle d. Which equation represents nuclear disintegration resulting in release of a beta particle. 1 0-1e 2 1 1H 3 2 1H 4 1 0n 22.
Which of the following represents an example of beta particle emission. The chemical symbol for a beta particle is the Greek letter beta. Aalpha emission Bgamma emission.
A neutron in the atom undergoes decay and will produce a proton electron the beta particle and an electron antineutrino. Which of the following correctly represents the transmutation wherein a curium-242 nucleus is bombarded with an alpha particle to produce a californium-245 nucleus. The beta particle is one of the particles that are emitted during radioactive decay.
A β particle is emitted a. The atomic number decreases by two and the mass number decrease by four b. O 98 6 104 15 8 1 o 87 85 239 93 Np-239N OB.
Physics 22062019 0300 iyanistacks50. Ignore the question on the image Answers. Answer Expert Verified 39 5 11 Erudite1 The beta particle is represented by the Greek letter β.
3 high speed neutrons are released to bombard other 235 v92 U isotopes. According to Reference Table N in the Reference Tables for Physical SettingChemistry a product of the radioactive decay of Ra-226 is. Which of the following symbols represents a beta particle.
E- or - or B- electrons or e or or B positrons. Which one of the following requires a particle. Which nuclide is represented by X.
Which of the symbols represent a beta particle. 1 Get Other questions on the subject. 2 high speed neutrons are released to bombard other 235 v92 U isotopes.

Physics Wallpaper Technology Physics And Chemistry Wallpaper Background 1575 X 1095 Id Physics Research Astrophysics Physics

Oneclass Which Symbol Represents A Beta Particle 0 1 E 0 1 E 1 0 N 0 0 Gamma 4 2 He Show Transcr

Alpha Decay Beta Decay Gamma Decay Electron Capture Positron Production Nuclear Chemistry Youtube

Question Video Identifying The Correct Equation For The Beta Decay Of Neptunium 239 Nagwa

Details Dietary Supplement Strength Size Recovery Micronized Gl3 L Glutamine Re Muscle Building Supplements Post Workout Recovery Bodybuilding Supplements

What Symbol Is Used For A Beta Particle Brainly Com

Beta Decay Https Scienceterms Net Physics Beta Decay Energy Forms Beta Particle Decay

Which Of The Following Represents A Beta Particle Brainly Com
Answered Which Of The Following Symbols Bartleby
Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry
Solved Which Of The Symbols Represent A Beta Particle Ob Oy Chegg Com
Alpha Particles Beta Particles Gamma Rays Positrons Electrons Protons And Neutrons Youtube

Radioactive Decay 2 Atomic Nucleus Mcat Content

Pin By Delights On Homeschool Science Nuclear Reaction Equations Nuclear

5 3 Types Of Radiation Chemistry Libretexts
What Is Conservation Law In Beta Decay Definition

Beta Decay Definition Examples Types Fermi S Theory Of Beta Decay
Comments
Post a Comment